Acrolite Meets ISO 13485:2016 Standards
Acrolite Inc is a contract manufacture of glass fiber optic lighting and sensing components and assemblies for the Medical Device Industry.
ISO 13485:2016 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer and applicable regulatory requirements. Such organizations can be involved in one or more stages of the life-cycle, including design and development, production, storage and distribution, installation, or servicing of a medical device and design and development or provision of associated activities (e.g. technical support). ISO 13485:2016 can also be used by suppliers or external parties that provide product, including quality management system-related services to such organizations.
Requirements of ISO 13485:2016 are applicable to organizations regardless of their size and regardless of their type except where explicitly stated. Wherever requirements are specified as applying to medical devices, the requirements apply equally to associated services as supplied by the organization.
The processes required by ISO 13485:2016 that are applicable to the organization, but are not performed by the organization, are the responsibility of the organization and are accounted for in the organization's quality management system by monitoring, maintaining, and controlling the processes.
If applicable regulatory requirements permit exclusions of design and development controls, this can be used as a justification for their exclusion from the quality management system. These regulatory requirements can provide alternative approaches that are to be addressed in the quality management system. It is the responsibility of the organization to ensure that claims of conformity to ISO 13485:2016 reflect any exclusion of design and development controls.
If any requirement in Clauses 6, 7 or 8 of ISO 13485:2016 is not applicable due to the activities undertaken by the organization or the nature of the medical device for which the quality management system is applied, the organization does not need to include such a requirement in its quality management system. For any clause that is determined to be not applicable, the organization records the justification as described in 4.2.2.
Acrolite Custom Fiber Optics
- Up to 20% > Light Transmission
- Greater Thermal Performance
- More consistent end termination
- Increased Resistance to Autoclaving
Market Applications
- Machine Vision
- Medical
- Dental
- Industrial Inspection
- Custom OEM Solutions
Acrofusion™ Bundled Fiber Optics
Through a proprietary process, Acrolite has developed a solution for transmitting up to 20% more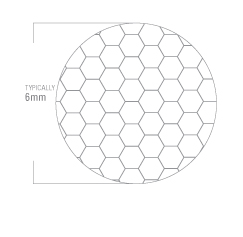 light through fiber optic cables. We call this process Acrofusion™.
light through fiber optic cables. We call this process Acrofusion™.
In a typical fiber optic cable assembly, space between each fiber strand equates to light-loss over the length of the cable. The longer the cable, the greater the loss.
Acrofusion™ greatly reduces this light loss by removing the space between individual fiber strands at the connector end, resulting in increased light output.
The diagram on the right shows a detail of a typical Acrofusion™ cable end
Because Acrofusion™ yields more light through the cable, that can improve the thermal performance of the assembly. The performance of our Acrofusion™ process is unmatched in the industry.

